The U.S. Department of Health and Human Services (HHS) is preparing to stop recommending routine COVID-19 vaccinations for children, teenagers, and pregnant women, according to confirmation from a senior agency official, as reported by GOP USA.
The change, first reported by The Wall Street Journal on May 15, marks a major policy shift from previous guidance issued during the pandemic.
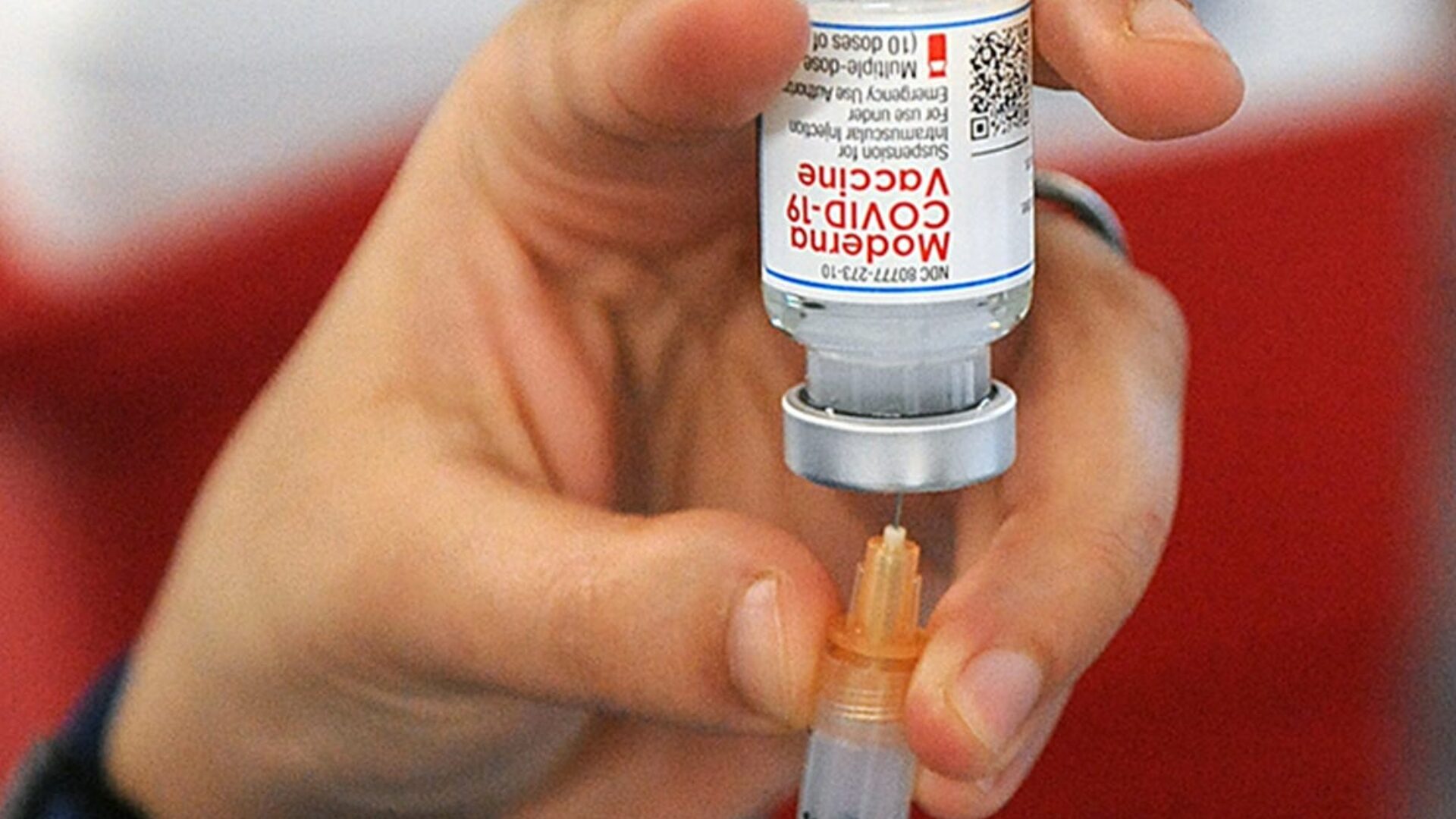
A formal announcement is expected in the coming days.
Trump’s Sovereign Wealth Fund: What Could It Mean For Your Money?
Currently, the Centers for Disease Control and Prevention (CDC) recommends COVID-19 vaccinations for everyone aged 6 months and older, using one of three approved formulations: Pfizer-BioNTech, Moderna (both mRNA-based), or Novavax’s protein-based shot.
The Food and Drug Administration (FDA) had been expected to rule on Novavax’s full approval by April 2, but that decision has been delayed.
The vaccine remains available only under emergency use authorization, which has a lower evidentiary requirement than full FDA approval.
According to CDC data, only 13 percent of children and 14 percent of pregnant women have received the most recent version of the COVID-19 shot.

This Could Be the Most Important Video Gun Owners Watch All Year
HHS Secretary Robert F. Kennedy Jr., who has a long history of raising concerns about vaccine safety, previously stated that Novavax’s vaccine has not demonstrated effectiveness.
Kennedy opposed COVID-19 vaccine mandates throughout the pandemic and, in 2021, petitioned the FDA to revoke emergency-use authorization for the shots altogether.
In 2023, the CDC added COVID-19 vaccines to the routine immunization schedule based on recommendations from its vaccine advisory panel. However, in a meeting last month, the same panel said it is now considering rolling back the universal recommendation.
During that meeting, officials also acknowledged that other countries, including the United Kingdom and Australia, do not advise healthy children to receive COVID-19 boosters.
FDA Commissioner Dr. Marty Makary echoed Kennedy’s skepticism about COVID-19 vaccine efficacy and safety. On May 15, Makary stated:
“Separate from my role as a regulator at the FDA, I am not encouraging or insisting young, healthy children to get a COVID shot unless there is new evidence that emerges that suggests there is a clear benefit.”
The FDA is expected to introduce a new framework for vaccine approvals next week, which will include stricter data requirements. According to HHS, all new vaccines will now be required to undergo placebo-controlled testing.
“We want to see vaccines that are available for high-risk individuals,” Makary added.
“And at the same time, we want some good science. We want some good clinical data.”
This move represents a significant policy pivot in the Biden-era public health approach to COVID-19 and comes as public trust in pandemic-era mandates continues to be re-evaluated.
Connect with Vetted Off-Duty Cops to Instantly Fulfill Your Security Needs
The opinions expressed by contributors and/or content partners are their own and do not necessarily reflect the views of LifeZette. Contact us for guidelines on submitting your own commentary.



![‘It’s a Recipe for a Hundred Years of National Dominance’: Stephen Miller [WATCH]](https://www.right2024.com/wp-content/uploads/2025/05/Stephen-Miller-Completely-Obliterates-CNN-Host-Over-Her-Illegal-Immigration-350x250.jpg)

![Trump Posts Hilarious Pope Meme, Leftists Immediately Melt Down [WATCH]](https://www.right2024.com/wp-content/uploads/2025/05/Trump-Posts-Hilarious-Pope-Meme-Leftists-Immediately-Melt-Down-WATCH-350x250.jpg)

![Wild Road Rage Brawl Erupts in Milwaukee [WATCH]](https://www.right2024.com/wp-content/uploads/2025/05/Road-Rage-Turns-Violent-in-Oregon-Minivan-Mows-Down-Motorcyclist-350x250.jpg)







